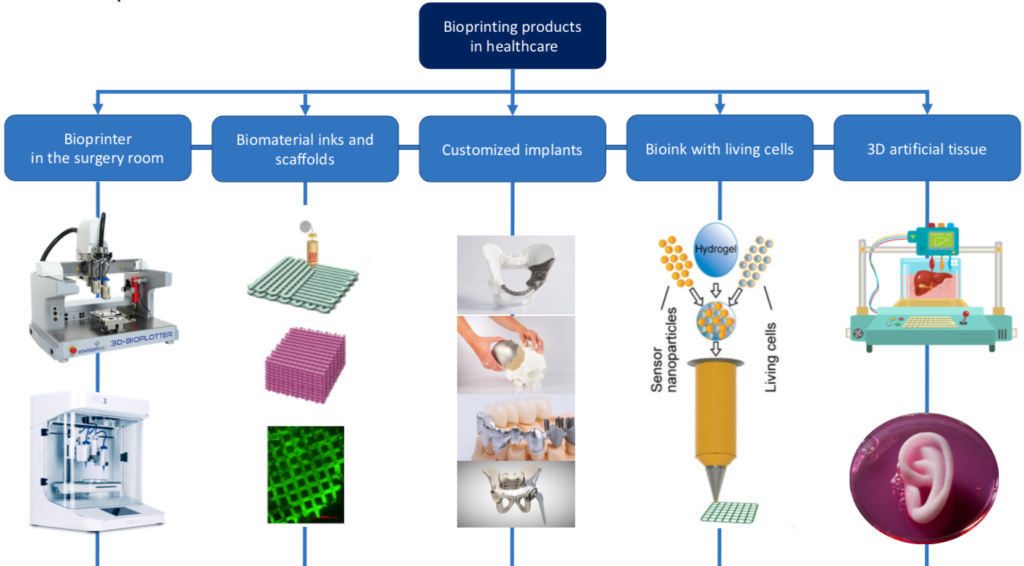
Every June, towards the end of academic year, the Industrial Engineering School at Universidad Politécnica de Madrid (ETSII-UPM) organizes a closure ceremony with all Master’s students and invited personalities from the UPM’s rectorate team and from the regional industry, so as to present engineering systems developed by student teams at ETSII-UPM within the Industriales Ingenia initiative. This year different UBORA devices, developed by UPM students within courses on “Bioengineering Design” (MSc in Industrial Engineering) and “MedTech” (MSc in Industrial Organization), in which the UBORA educational model is applied, have been presented. The UBORA e-infrastructure has been used, for guiding such developments, and the UBORA tool for medical device classification has been employed, for training students on the relevance of using standards for reaching successful and safe medical devices. These courses have been coordinated by Prof. Andrés Díaz Lantada (Bioengineering Design) and by Prof. Luis Ignacio Ballesteros (MedTech), both UBORA professors and mentors and will serve as cases of study for UBORA Design Schools and are shared through the UBORA e-infrastructure.

In the image above, UBORA device “Lazarus Biotech Standing Frame”, a standing frame for children with mobility problems developed according to a need assessment performed in collaboration with ASPADIR foundation, which works with children with physical and sensory disabilities.