On February 6th, Carmelo De Maria and Licia Di Pietro were hosted by WeMake within the cycle of webinars on digital social innovation, in the framework of DSI4EU project.
During the webinar, Licia and Carmelo, covered a crucial issue for those who in the world of Makers and Fablabs are dealing with the development of medical devices. The main contents of the new European Regulation on Medical Devices was presented in a simplified way with particular attention to their classification and the procedures envisaged for CE marking.
Moreover, they presented several case studies developed using the UBORA platform in order to demonstrate the easy to use of the e-infrastructure to design medical devices compliant to the current European Regulation.
Starting from the webinar, WeMake team, with the help of the UBORA team developed the infographic “Open Source Medical Devices — a visual guide for makers”. This guide aims to provide the Do-It-Yourself communities with an easy-to-use and step-by-step documentation on how to go from a prototype of an open care device to a product that can be compliant with regulations and, therefore, ready to get to the market.
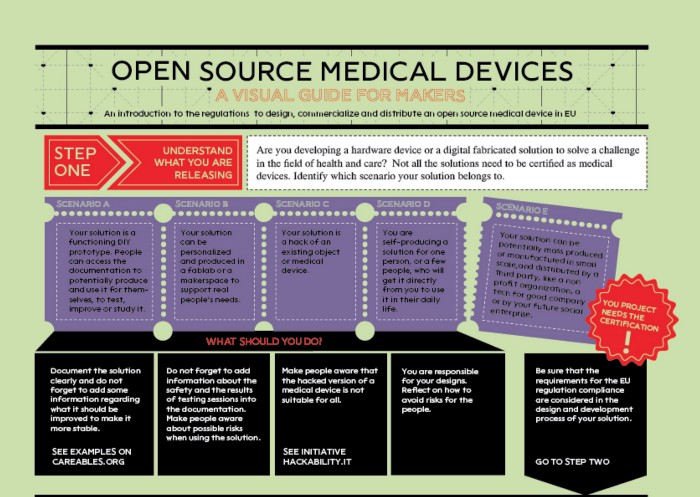
Documentation:
