WHO estimates that today, more than 1 billion people need one or more assistive products. With a global ageing population and rise in noncommunicable diseases, this number will rise beyond 2 billion by 2050, with many older people needing two or more products as they age.
Currently, only one in ten people in need have access. To address the substantial gap between the need for and provision of assistive technology, WHO established the Global Cooperation on Assistive Technology (GATE).

Licia Di Pietro, UBORA team member, is spending a period of 3 months at the WHO Headquarters in Geneva as an intern. She is involved in the activities of supporting the work of the GATE based in the Department of Essential Medicines and Health Products.
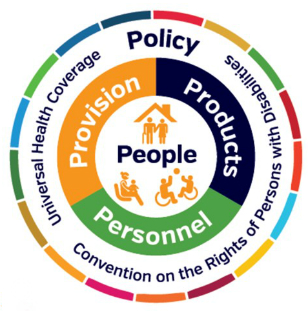
The GATE initiative has only one goal: to improve access to high-quality affordable assistive products globally. To achieve this, the GATE initiative is focusing on five interlinked areas (5P): people, policy, products, provision and personnel.
During her internship, Licia is supporting the development of Assistive Product Specifications and mapping need for assistive technology using existing population data sources. In particular, she is involved in a study conducted between WHO Headquarters and WHO EURO, where WHO Headquarters led the estimation of the prevalence of functional limitations across all 4 countries and mapping potential AP of benefit and WHO EURO led the implementation and follow up of the rapid AT capacity assessment.