The certification of a medical device can require animal testing: norms and ethical, and economic reasons impose a careful understanding of the mandatory steps for ensuring safety and efficacy of new technologies
In this context, the European Union Directive 2010/63 / EU on the protection of animals used for scientific purposes constitute a milestone, but many times there is no clear understanding of who-is-what, leading to useless experiments.
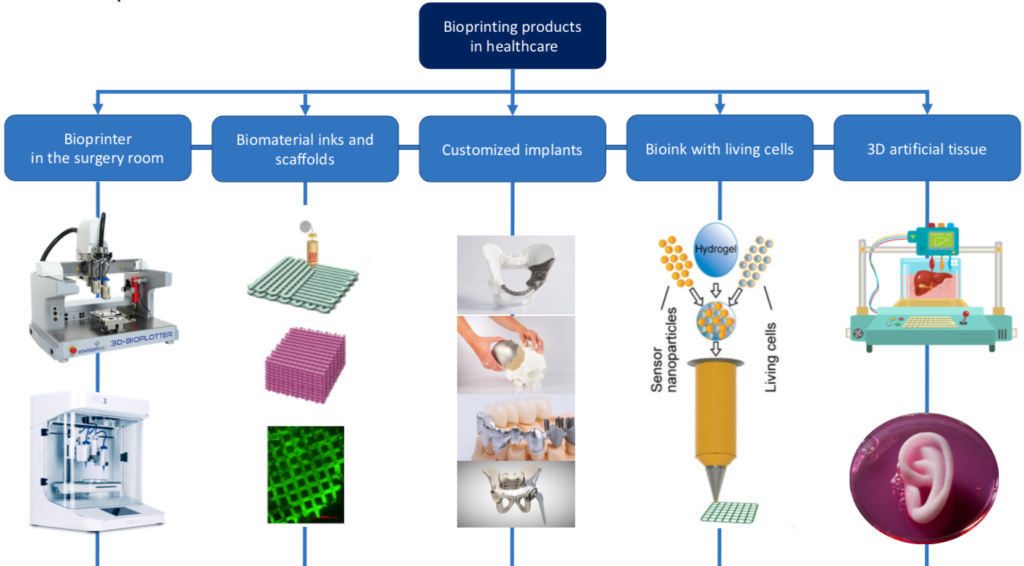
The UBORA team gave its contribution to the discussion, try to make a clarification on one of the cutting edge fields of research, Bioprinting of scaffold and living tissues, where a clear distinction between medical devices and advanced therapies medicinal products can be a first step toward the refinement of animal testing.